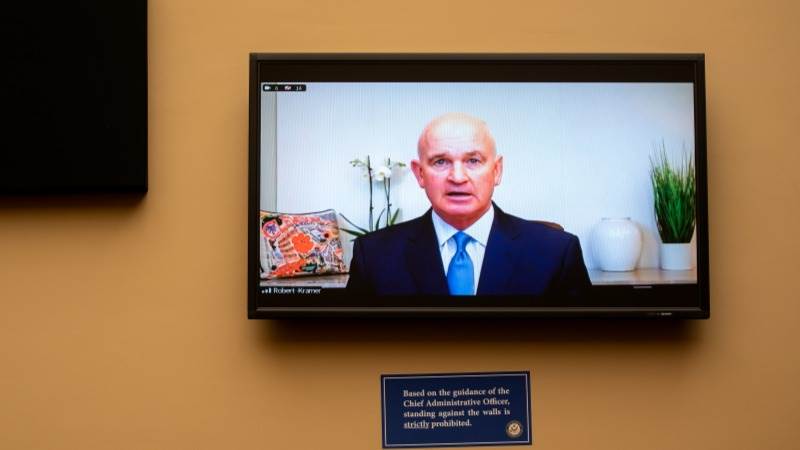
President and Chief Executive Officer of Emergent BioSolutions Robert Kramer stated on Wednesday that over 100 million Johnson and Johnson (J&J) COVID-19 vaccine doses are currently being held up by the Food and Drug Administration (FDA) while it investigates their safety after cross-contamination was found in Emergent's plant that manufactures ingredients for both the J&J and AstraZeneca jabs.
"The FDA is evaluating, to my understanding, the doses that had been manufactured for bulk drug substances, most of which has been provided to J&J," Kramer said at a House Select Subcommittee on the Coronavirus Crisis hearing. "These vaccines have passed all of our internal quality control measures," he assured later in the hearing referring to the doses under review.
An ingredient mix-up between the two different vaccines reported earlier this year at the company's Baltimore plant resulted in 15 million jabs being discarded. Kramer also stated at the hearing that production of the J&J vaccine in the US could "resume [..] within a matter of days."